Risk analysis (or impact analysis) is required for any CAPA life cycle and involves the identification, Examination and prioritization of risks, and the subsequent application of effort to minimize, monitor, and control the probability and/or impact of a negative outcome.
Every Organization can conduct its own assessment by incorporating a simple CAPA risk assessment Matrix-Table by following these three steps:
- Checking of historical data to assess risk attributes, frequency at which they were occurring and its impact analysis. From this vital data any organization can reasonably determine, companies quality system risk exposure along with its frequency of its reoccurrence as well as risk tolerance.
- After collecting the vital information regards risks involved and its frequency the organization has to quantify its impact on quality using well established and scientific and transparent Methodology.
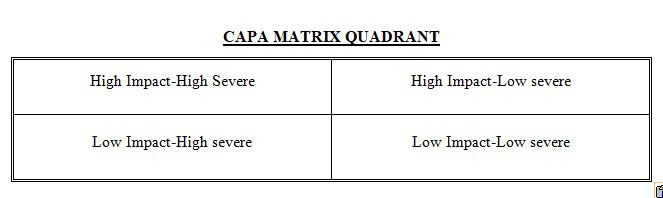
- Now it the third step involves a Plotting of n x n matrix, which involves (frequency versus severity (major, minor)) to Priotarize on the High-risk and High-impact category.
Utilization of a Tabel-matrix approach can, subsequently, allows certain activities to be triggered automatically based on risk such that the quality of the product is adequately protected.
This CAPA risk matrix becomes a benchmark tool that allows you to determine and priotarize the corresponding actions to events based on those criteria, including no action if it falls within the acceptable risk category.
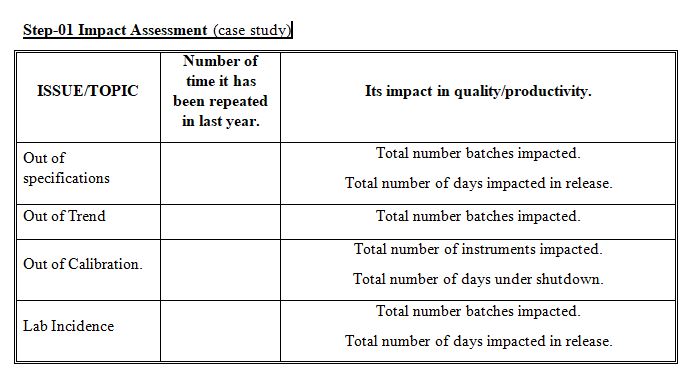
Step-2
Classify the Issues/Topics based on its impact productivity of organization. Such as High Risk, Low Risk based on the data generated last year. (Total number of days, Total number of batches…etc)
Step-3
Classify the Issues/Topics based on its impact on product quality of organization. Such as High severe, Low severity based on the data generated last year. OOS could be high severe because it directly impacts quality Documentation, Right first time parameter could be low severe because it does not directly impacts quality.
Step-4
After collecting total data on various issues and frequency with which they are reoccurring, we have classified Topics into following categories:
- High Impact-High Severe: Topics which are severe and occur most frequently.
- High Impact-Low severe: Topics which are less-severe and occur most frequently.
- Low Impact-High severe: Topics which are severe and occur less frequently.
- Low Impact-Low severe: Topics which are less severe and occur less frequently.