Document control refers to the process for approving, sharing, and editing a report as well as designating a person or people to carry out those tactics. Any laboratory must have a successful document control process, therefore it’s critical to understand the several potential problems that could arise from improper management. Details of five distinct phases—from data production to obsolescence—will be covered in this article. We’ll talk about how crucial it is to set up explicit policies and processes for document control and how having a secure document repository is essential.
Finally, we will discuss the critical steps needed to ensure the efficiency, reliability, and security of document control processes.
Document control comprises the following five intermediate steps:
- Document Creation
- Approval
- Change Control
- Retrieval
- Obsolescence
Step 1: Document Creation
Documents creation effort is Team effort comprising of two or more departments, coordination between them is essential for quality document. Further creation of documents is cumbersome, tedious and time consuming. Report creation quite often requires joint effort, which could be tedious without the proper devices and a compelling framework.
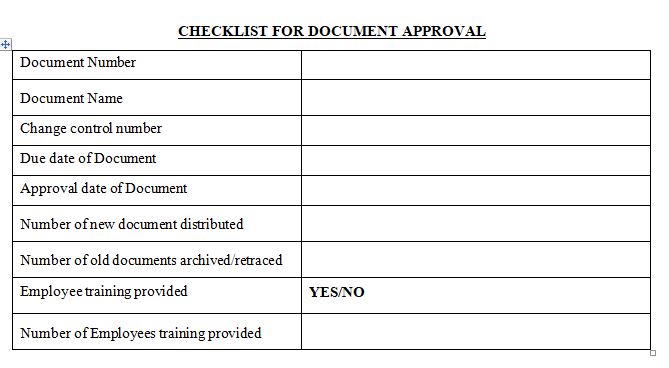
Stage 2: Document Approval and Distribution
Postponements can make the cycle come to a standstill. This occurs if the cycle depends on the presence of approvers/reviewers and it doesn’t give a component to deal with their nonattendance.
Apparition of updates/ revisions past haunts users. This happens when old modifications are uncontrolled and continue to keep on surfacing.
Employee awareness and updating Training can be forgotten and get lost in noise. This is bound to happen if after document is approved, then necessary training to its related employees is not triggered.
Stage 3: Review and Change Control
Document control can be ignored. This happens document preparation cycle fails to integrate a standard review to ensure that a document is still applicable and updated after certain period.
Documentation is Team effort comprising of two or more departments, coordination between them is essential for quality document. Ensuring the right people are involved in every step is a big challenge.
Stage 4: Retrieval
Endorsed documents must be easy to track down and cited. Else they were of no use, it should easily available to the concerned persons for reference.
Stage 5: Obsolescence
Quality Assurance department should have proper archival maintenance policy. Retention of documents will be ineffective if a well-designed retention policy is not put in place and enforced.
Audit, Inspection and Regulatory requirements will direct organization retention time periods. Further organization’s Archival maintenance and retrieval policies should be based on risk-based approach to ascertain sensible document retention periods for various types of documents.
After reading this blog, we hope you can understand the importance documentation errors and properly use the proposed checklist and improve on it further.
Now that you know this:- hopefully you are in position to answer the Assignment(s)
Do QA department have SOP for archival of documents, Change control procedures for change/revision/updating of documents?
In your lab, documents such as IQ/OQ/PQ, Analyst qualification and Vendors are properly archived? Further easily retrievable at the time of requirement?
Do QA department have SOP for timely review and updating of documents such as SOPs, STP?
Do QA department provide proper and timely training to employees after relevant documents’ such as SOP, STP are revised?