For any laboratory or organization, document control is a crucial procedure. Document control is a fundamental quality process with numerous difficulties, which might potentially lead to noncompliance. It is the system that makes that company-related papers are correct, secure, and up to date. Nonetheless, handling documents can frequently be difficult, so it’s critical to understand the typical problems that might occur. The following are three of the most common issues that affect quality compliance involving document control will be covered in this post
Long document Time Periods due to inefficient distribution, review, and approval of documents. When the quality departments create documents, they must route those files for review and approval, follow-up is also processed by email or phone or personally. Once endorsed; printed in hard copy and stored in Archival room under supervision for future reference. It is reasonably difficult to manage hundreds or thousands of documents, especially if they undergo multiple revisions and regular updates, hence sufficient staff and resources should be allotted.
Lack of control in the change control process.
Apparition of updates/ revisions past haunts users. This happens when old modifications are uncontrolled and continue to keep on surfacing.
This bound to happen when paper copies of old SOPs/ outdated SOPs/GTP/STPs and other procedures continue to found in work space, this happens because of lack of effective document retrieval and archival policies
Document review should be part of organizational change control process. A standardized, time bound and periodic document review process can help ensure necessary changes have been documented and that the actual process and the documented process are in sync.
Document control can be ignored. This happens document preparation cycle fails to integrate a standard review to ensure that a document is still applicable and updated after certain period.
Documentation is Team effort comprising of two or more departments, coordination between them is essential for quality document. Ensuring the right people are involved in every step is a big challenge.
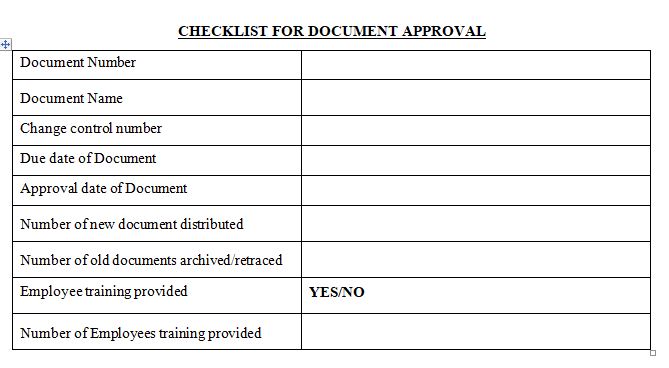
Training: Employee awareness and updating Training can be forgotten and get lost in noise. This is bound to happen if after document is approved, the necessary training to its related employees is not triggered.
Once a quality document is approved, affected employees should be notified and provided access to the document so they can be trained on the document prior to its effective date.
After reading this blog, we hope you can understand the importance documentation errors and properly use the proposed checklist and improve on it further.
Now that you know this:- hopefully you are in position to answer the Assignment(s)
Do QA department provide proper and timely training to employees after relevant documents’ such as SOP, STP are revised?
Do QA department have SOPs for archival of documents and Change control procedures for change/revision/updating of documents?
Can you specify few bottlenecks you have faced during creation and approval of documents? Can you propose better solutions for smooth procedures for creation and approval of documents?
Can you specify any communication problems faced between your lab personal and QA departments?