Change control in the analytical laboratory is a lengthy and multi-departmental process. Knowing when formal change control is needed and what it requires can streamline the process and reduce waste.
1. Laboratory Instrument Change Control
Change control for laboratory instruments may or may not be required depending on mitigating circumstances. Instrument change control is recommended when
1) instruments or components are moved,
2) firmware is upgraded, and
3) systems or components are retired.
However, when you move an instrument within the same lab, move an instrument that has been designated as “portable” (instruments on carts e.g. LCs used with multiple mass spec systems, electronic pipettes, weight sets, etc.), and undergo preventative maintenance, instrument change control is not required. Meanwhile, an instrument should be recalibrated after it has been moved.
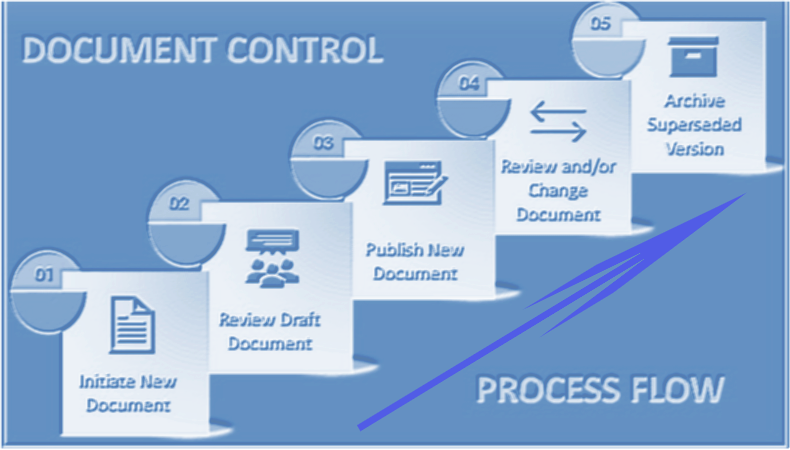
2. Laboratory Document Change Control
Internal procedures and federal regulations should be used as basis for changing laboratory documents. Small, typographical changes do not require change control unless specified in procedures. However, typographical changes that impact processes or data should follow a change control process.
3. Analytical Method Change Control
When implementing change control for a validated analytical method, a good means of tracking background information for changes is required. Supporting documents or data supporting the change needs to be included in the change request.
When changing a non-validated method, change control is not typically required, although it must be specified in the procedures.