For what reason is documentation such a major issue? There are two fundamental reasons.
First reason being pharma-industries regulated by FDA requires immense measure of documentation.
Secondly, organizations regularly don’t move toward documentation efficiently.
Think about the ideal documentation plan as satisfying the five Cs—it must be clear, correct, concise, coherent and Consistent.
Here are following proposals to produce clear, correct, concise, coherent and Consistent documents.
Resolve to ensure enough infrastructures are accessible to finish documentation at an elevated level.
Documentation is Team effort comprising of two or more departments, coordination between them is essential for quality document. Ensuring the right people are involved in every step is a big challenge.
Conduct an intensive examination of your current documentation and your requirements for new or overhauled documents.
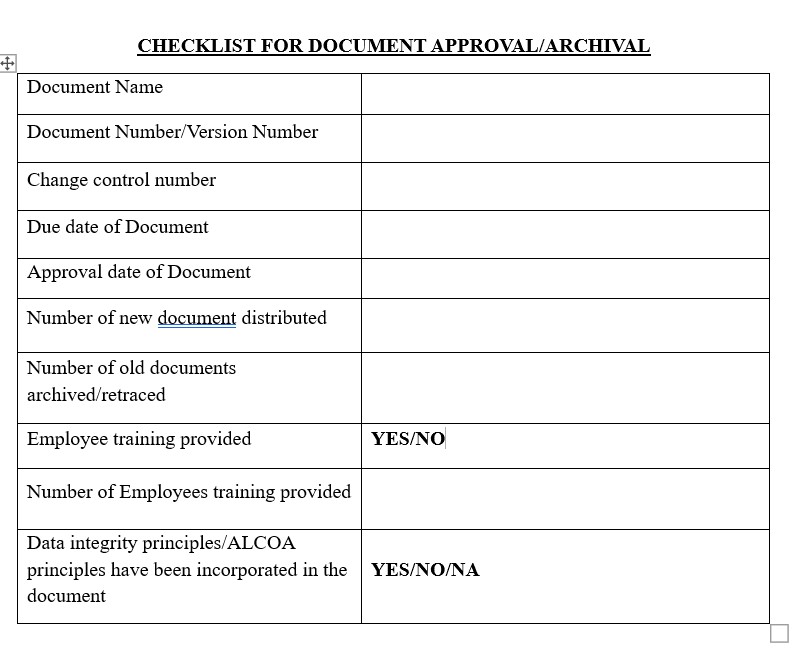
Endorsed documents must be easy to track down and cited. Else they were of no use; it should easily available to the concerned persons for reference. Quality Assurance department should have proper archival maintenance policy.
Lacking a comprehensive documentation will lead unnecessary duplication. In addition, unhelpful documents enormously complicate training. It’s so much better to have real-time mentors who really utilize the documentation that exists and necessitate that their trainees use it too. However a precondition exists, that documents developed must be legible and implementable.
You spend a lot of time and effort to make sure your product is safe and effective and that it passes regulatory scrutiny. Your documentation needs the similar degree of consideration.
After ensuring the necessary infrastructure is provided, it is necessary to be managed logically.
Document review should be part of organizational change control process. A standardized, time bound and periodic document review process can help ensure necessary changes have been documented and that the actual process and the documented process are in sync.
Once a quality document is approved, affected employees should be notified and provided access to the document so they can be trained on the document prior to its effective date.
Implementing Document Control methods or procedures
Every document should be accompanied with:
- Revision history: For any record, you should have the option to see when it was given, when it was modified, reason of change and who made which changes.
- Document recall: When a new report supplements an old one, organization should document retrieval procedure of all the old copies.
- Document Safeguards: What controls do you have set up to keep individuals from erasing or adjusting records? Authorizations should be set up that keep unapproved people from changing key records, for example, SOPs, particulars and testing techniques.
- Incorporating Data Integrity in Documents
The Auditing/Regulatory agencies expects data to be attributable, legible, and complete, an original or true copy and accurate (ALCOA). ALCOA has been introduced by FDA while ALCOA+ concept has been introduced by European MHRA. - Control Paper Records
Accessibility and availability to blank paper formats for raw/source data recording should be appropriately controlled and monitored. All blank formats issued by quality assurance department should be appropriately numbered and should have format number and should be monitored by issuance register.
Use of controlled books with numbered pages, may be necessary to prevent the re-creation of a record; while retaining incomplete forms and recording why they were replaced.
After reading this blog, we hope you can understand the importance documentation errors and properly use the proposed checklist and improve on it further.
Now that you know this:- hopefully you are in position to answer the Assignment(s)
Do QA department have SOP for archival of documents, Change control procedures for change/revision/updating of documents?
Do QA department have SOP for timely review and updating of documents such as SOPs, STP?
Do QA department provide proper and timely training to employees after relevant documents’ such as SOP, STP are revised?
Can you specify few bottlenecks you have faced during creation and approval of documents? Can you propose better solutions for smooth procedures for creation and approval of documents?
Can you specify any communication problems faced between your lab personal and QA departments?
What are ALCOA Principles? Are your departments documentation practices incorporated on ALCOA Principles?
Have you found any loose sheets/blank sheets in uncontrolled manner in your lab? Please immediately report to concerned manager?
Have you found any books for documentation in uncontrolled manner in your lab? Please immediately report to concerned manager?