Operational qualification (OQ) is carried out after every protocol of the IQ phase has been met to verify that the device’s overall performance is consistent with the consumer requirement specification within the manufacturer’s specified operating ranges.
The OQ cycle does the following:
- Identifies the vital working parameters.
- Identify the lists the experiments to be conducted on the vital working parameters.
- Plan the sequence of experiments to be conducted and ensures the proposed acceptance criteria of the product were met.
The principle motivation of OQ is to become aware of and check out the capabilities of the equipment that can impact the product quality.
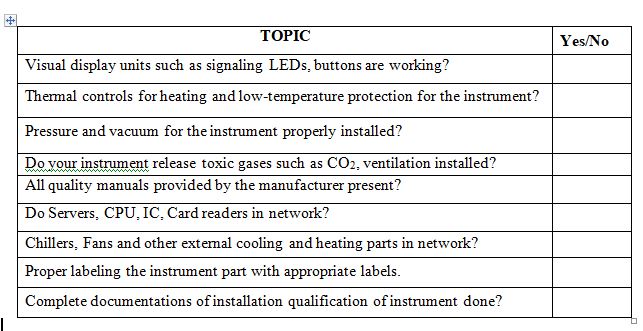
- Visual display units such as signaling LEDs, buttons.
- Thermal controls for overheating and low-temperature protection for the systems.
- The Pressure and vacuum fluctuations controlling frameworks.
- Effluent and toxic gases evacuations control framework such as CO2.
- Lighting and Humidity observations and controlling framework.
- Chillers, Fans and other external speed controlling framework.
- Servers, CPU, IC, Card readers and access controlling framework.
By and large, we will begin the OQ cycle as per plan and let it arrive at standard working conditions. The laboratory personnel will then observe the operating parameters to ensure that the process is stable and working as expected.
While the OQ is being directed, you’ll need to carry-out numerous other tests to make sure they were performing within the indicated range. From the data obtained from these tests will guide to incorporate process controls, voltage and amperage controls, computer and software systems, ecological conditions (e.g., temperature, humidity, air quality, etc.) for optimum operational performance of the system.
After reading this blog, we hope you can understand the importance of Installation qualification and properly use the proposed checklist and improve on it further.
Now that you know this:- hopefully you are in position to answer the Assignment(s)
Please review the IQ/OQ/PQ of recently installed equipment in your laboratory?
In your lab, do IQ/OQ/PQ are properly archived? Further easily retrievable at the time of requirement (especially during audits)?
In your lab, quality manuals submitted by Vendor is stored properly and accessible to analyst at the time of requirements?
Does SOP design based on IQ/OQ/PQ of the instrument?
Does SOP incorporate preventive maintenance and calibration as per IQ/OQ/PQ or vendor recommendations?
Is training of laboratory staff part of new IQ/OQ/PQ?