This article discusses the importance of Equipment maintenance, calibration, and workplace optimization practices and the benefits it can provide.
Service and calibration of lab equipment are included in your inventory management procedure. Reducing waste and the expense of repairs or replacement is achieved by maintaining your equipment. Additionally, it makes working in your laboratory safer. Your workplace optimisation practises also include equipment maintenance, calibration, and optimization. Examine the upkeep and adherence to equipment maintenance schedules each time you audit your lab.
Make a list of all the equipment that needs maintenance, and then draw together a schedule based on the advice of the manufacturer. Keep the checklist close by where you keep your equipment, and use it whenever you are doing inventory audits. This will serve as a reminder to stay current with service and a notification of approaching schedules.
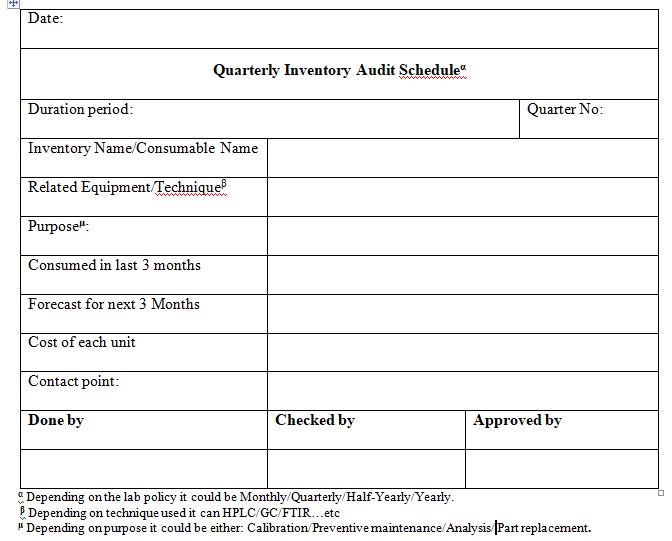
Develop Inventory Audit Schedules
Depending on the size of the lab and the importance of the lab inventory, stocking level audits are performed on a monthly, quarterly, and annual basis. Additionally, managers need to check their records to make sure everything is in order. Managers need to make sure that no invoices are missing, monitor data collection, and double-check signatures.
Verify that inventory levels are being managed properly, that there is no expensive waste, that records are being kept properly, that equipment maintenance requirements are being met, and that supply expiration dates are being observed.
Use this chance to check that your testing supplies are kept in the right conditions for light and temperature. For testing on the microbiology of the water supply, this can be extremely important.
Review your lab’s safety manual as well as any compliance guidelines for storage, and take any necessary adjustments.
In conclusion, maintain equipment maintenance schedules and Conduct stocking level audits on a weekly, quarterly, and annual basis.