In the pharma manufacturing, change alludes to any adjustments in materials, facilities, utilities, product design, formulations, processes, packing/labeling, equipment, computer systems, including all its related documents such as SOPs, STPs, etc.
A change control system is used to handle a relatively small/simple adjustment, such as a part replacement in an manufacturing equipment/analytical instruments in the laboratory or an version revision of existing document or creation of new documents, otherwise it change control system can also be used to rectify/close an issue serious enough to shut down production.
The way that change is unavoidable makes control a basic factor, particularly in FDA and other regulatory conditions, where inappropriate or “uncontrolled” changes could affect the safety and efficacy of products and directly impact public health and safety.
Challenges during execution of Change Control Systems and Their possible rectifications
Correspondence: Correspondence breakdown is perhaps the most well-known challenge, especially for organizations that rely on tribal knowledge or classical paper-based systems to manage change. Regardless of whether the breakdown displays as lack of timely follow up, objective delays in completion of work or completion of work in wrong or erroneous way thus comprehensively increasing the risk of cGLP and cGMP noncompliance.
Updating of Documents: After Correspondence or communication gap in the organization, the second most important challenge regarding change control is documentation, such as timely and frequently updated old documents with new updated versions which is profoundly inclined to human error.
Total Time Required: The total time required to initiate, follow-up, track and execute change control, should be minimum to make change effective. Hence online tracking system should be implemented to control Total turnaround time.
Training Challenges: When change happens, you are needed to prepare all representatives who will be influenced by the change. When proper training is isolated outside the change request framework, for what it’s worth in a run of the mill paper-based framework, changes in SOPs, strategies, and different archives frequently become lost despite any effort to the contrary.
Thus defining what is change control, ways and tools to control and execute change control for small/minor or major changes is a must for every organization to increase customer satisfaction and prevent expensive and humiliating product recalls and cGLP, cGMP violations.
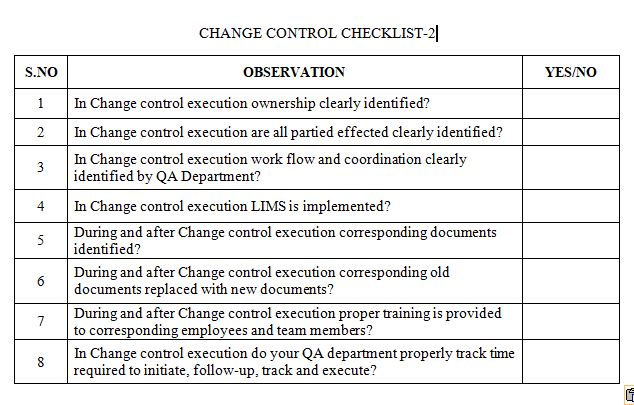
After reading this blog, we hope you can understand the importance of change control and properly use the proposed checklist and improve on it further.
Now that you know this:- hopefully you are in position to answer the Assignment(s)
Have you been involved in any change control activities in your lab? If yes recollect your activities?
Does your lab have proper SOP for the implementation of change control in your lab? If so are there effective?
Do you clearly identify whether proposed changed is Critical/Major/Minor? If yes what is the criteria?
Do you clearly identify whether proposed changes effect the quality of the product? Stability of the product? If so then it is properly captured and its impact properly assessed?
What is average time period required to properly close change control in your lab? Can you suggest any 3 measures to improve the quality of change control?
Have you found any communication gaps between different departments to smoothly close change control system?