Human Error in CAPA (Corrective Action Preventive Action) is an important factor to consider when dealing with root cause analysis and quality management. This article will discuss the various aspects of human error and its contribution to the effectiveness of CAPA, including how to identify, assess, and address human error in the context of CAPA.
When human mistake is suggested more regularly than it is expected to occur, it gives inference to reviewers that problems aren’t being reviewed for Root Cause Analysis, organization isn’t allotting required time and resources (this is just an example or case study).
In another example, quality issues which occur (generally one-time errors), by a well-trained staff worker, following a well defined SOP-driven process.
We would try to answer these two extreme examples, by Human error factor. In this present situation, it is useful to equip our-self with a Human Error model for analyzing what might appear to be human errors in order to determine whether those actions were intentional or incidental. Thus error can classified as 1) Intentional 2) Incidental.
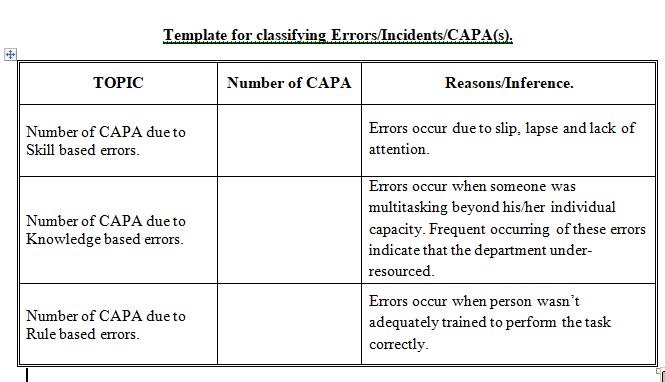
Thus Human Error Model, errors that are shown to be “Intentional” can be considered genuinely “human,” which then classified into one of three categories: 1) Skill-based, 2) Rule-based, and 3) Knowledge-based mistakes.
- Skill-based: Those that are skill-based can be either a slip or a lapse. Both of these points to the same root cause: a lack of attention, it’s important to note that these types of errors should not be happening frequently.
- Knowledge-based Errors
These types of errors occur when someone was multitasking beyond his/her individual Capacity, most important reasons for Knowledge base errors might be due to:
- Multi-Tasking of the Analyst/chemist, this is performing more than one task simultaneously?
- Improper distribution of work by supervisor, which leads to multi-tasking by individuals beyond their limit
- Frequent occurring of these errors indicate that the department under-resourced by Human capital.
- Rule-based Errors
These errors are more technical in nature, such as someone applying an incorrect rounding rule which ultimately generates an out-of-specification (OOS) result. In this situation, question why the person wasn’t adequately prepared to perform the task correctly.
- Did the analyst/chemist receive sufficient training before performing the activity?
- Did practice actually reflect the operations they were performing on the floor
- Does the procedure specify the details to the degree they need to be explained in order to be performed correctly?
Now that you know this:- hopefully you are in position to answer the Assignment(s)
- What is percentage of CAPA attributed to Human errors? Increase of CAPA attributed to Human errors, gives inference to reviewers that problems aren’t being reviewed for Root Cause Analysis?
- How can a Human Error model be used to analyze and classify different types of human errors? Please analyze recently initiated CAPA and classify the errors?
In conclusion, human error is a significant aspect to consider when implementing CAPA in root cause analysis and quality management. It is crucial to understand that humans are prone to make mistakes, and these errors can have a significant impact on the effectiveness of CAPA. By recognizing and addressing human error, organizations can improve their processes, strengthen their quality management systems, and ultimately achieve their objectives. By prioritizing human error management and incorporating it into the CAPA system, organizations can improve their overall performance and enhance customer satisfaction.