The crucial task of ensuring that medications and medical equipment are suitable for use by the general population falls to the Food and Drug Administration (FDA). As a result, they demand that businesses follow their regulatory standards when producing these goods. Companies must abide by these rules in order to receive FDA approval and maintain a good reputation. Adopting sound Computer System Validation (CSV) procedures is one of the most crucial steps in guaranteeing compliance. CSV procedures entail the use of modern software to validate and record the product’s safety and efficacy. Companies can prevent FDA observations and guarantee that their products fulfil the FDA’s guidelines by adhering to good CSV practises.
Observation: Failure to Validate a Regulated System
Computer System Validation (CSV) is an integral part of demonstrating regulatory compliance since it provides confirmation that computer systems function according to their intended use.
In the case of Computer System Validation, an inspector would typically look for documented evidence that validation activities were performed in accordance with approved validation procedures.
Observation: Absence of Written Procedures
Procedures provide an established, approved framework for carrying out tasks within a regulated environment. Additional procedures pertaining to regulated computer systems are necessary to meet the procedural control requirements of 21 CFR Part 11. These procedures should cover IT-related activities, such as System Maintenance and Logical Security. Procedures should also be in place to manage incidents involving GxP electronic systems along with any changes made to these systems
Observation: Written Procedures Not Followed
It is important to be able to demonstrate that the written procedures in place are followed over the course of day-to-day activities. When performing computer system validation, make sure that any deviations to the validation plan are documented and a rationale for the deviation is provided. Validation non-conformances should be addressed by identifying the root cause and implementing appropriate corrective actions. The system must not be released for use prior to completing validation and any system limitations should be specified in writing. Changes made to the system once it is validated should be put in place in accordance with an approved change request.
Observation: Failure to Maintain Records
Computer systems are used to manage a variety of regulated documents, ranging from training records and complaint files to raw data obtained during laboratory and production activities. Employing a risk-based approach when validating a computer system in a GxP environment can prevent potential issues involving document management and data retrieval. The validation should verify that provisions are in place for systemic data back-up, restoration and archiving. If the computer system allows for the use of electronic signatures, these signatures should be shown to be Part 11 compliant during validation.
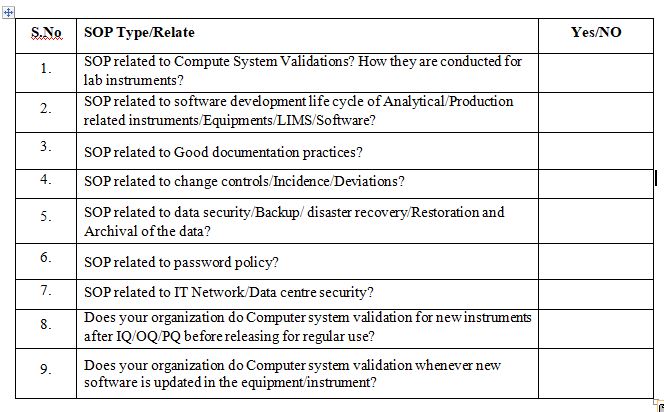
Do you have the following SOP’s closely related to Computer System Validation?
After reading this blog, we hope you can understand the importance of computer system validation and properly use the proposed checklist and improve on it further.
Now that you know this:- hopefully you are in position to answer the Assignment(s)
- Please review the Computer system validation of recently installed equipment in your laboratory?
- In your lab, do computer system validation properly archived? Further easily retrievable at the time of requirement?
- Do your organization have the following SOP related to Computer system validation? Password policy? Data backup/Security/Archival/Disaster recovery and E-signs?
- Please review SOPs related to Computer system validation? Password policy? Data backup/Security/Archival/Disaster recovery and E-signs, do you find any gaps in the framing of these SOPs please highlight the same and bring them to the notice of QA department?