PQ is the last step in qualification approaches for instrument, and this step entails confirming and documenting that the instrument is performing reproducibly within predetermined specified range. Rather than checking out every part and subpart individually, they’re all examined collectively as a part of qualification process.
PQ checklist is used to ensure that the system or product meets all of the criteria specified in the requirements document. The checklist can also be used to identify any areas of improvement that should be addressed before the product is released. This article will provide an overview of PQ checklists and how they are used to ensure the successful deployment of a system or product.
Important aspects of a PQ Protocol
Here are some elements that should be incorporated in your PQ protocol:
- Elaborate description of the methodology in the routine work environment.
- Acceptable time duration of the PQ (dependent on the nature of the process)
- Providing Standard operating parameters and Acceptance criteria for qualification.
- Provide clear methodology for every aspect of testing.
- Test information to be collected and the time-period for collection of the same.
- Instructions on how experiment data were to be reported, including clear procedures for handling abnormal data from unexpected results.
- Provide list of statistical tools that could be used to collation, analyzing and summarizing the data.
Contents of the Performance Qualification Report
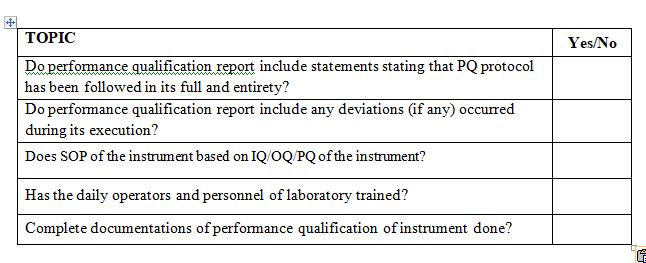
Instruments Performance Qualification report must include statements stating that PQ protocol has been followed in its full and entirety it should mention any deviations during execution of performance qualification.
Report should provide reference for the information collected during experiments.
Report should provide summary of conclusions, if any deviations occurred remediation measures taken.
Has the SOP been established and maintained, defining nominal values for method parameters.
Have the operators been trained sufficiently and verified?
Is there a process in place to evaluate proposed process changes to see if the process requires revalidation?
Now that you know this:- hopefully you are in position to answer the Assignment(s)
- Please review the IQ/OQ/PQ of recently installed equipment in your laboratory?
- In your lab, do IQ/OQ/PQ are properly archived? Further easily retrievable at the time of requirement?
- In your lab, quality manuals submitted by Vendor is stored properly and accessible to analyst at the time of requirements?
- Does SOP design based on IQ/OQ/PQ of the instrument?
- Does SOP incorporate preventive maintenance and calibration as per IQ/OQ/PQ or vendor recommendations?
- Is training of laboratory staff part of new IQ/OQ/PQ?
- After reading this blog, we hope you can understand the importance of operation qualification and properly use the proposed checklist and improve on it further.