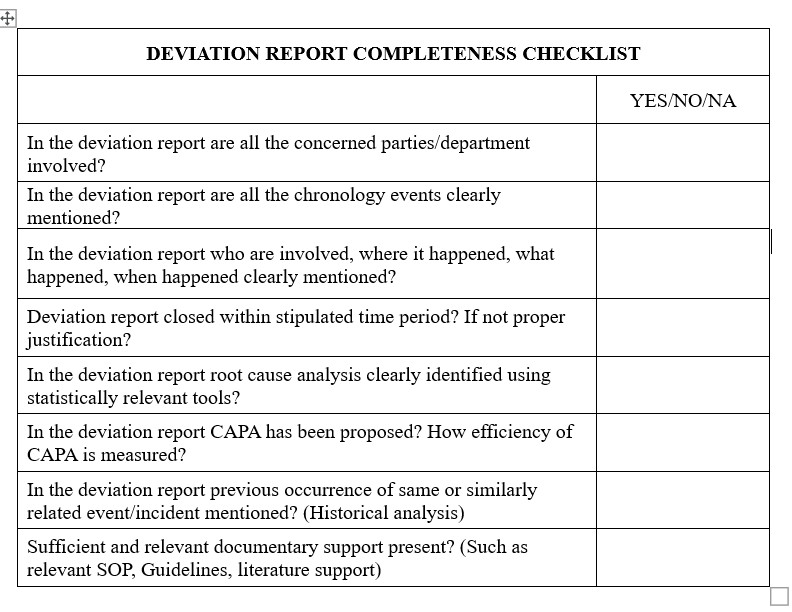
A successful corrective and preventative action (CAPA) process requires deviation investigations. They assist organizations in locating the primary reason of a deviation, dealing with it, and putting preventative measures in place. It’s not always easy to carry out efficient examinations of deviations, though. To make sure their CAPA process is actually effective; organizations need to be aware of a few frequent problems. This post will examine seven typical mistakes that might reduce the efficacy of deviation investigations and offer advice on how to prevent them. Organizations can promote continual improvement in their operations and raise the general caliber of their CAPA process by being aware of and taking action against these problems.
The accompanying segments recognize basic missteps organizations make while investigating deviation – and how organizations can reduce them.
1. Underutilization of historical data for continuous improvement
The information curated over the long history of the organization through investigations contains a wealth of data, which can be utilized for simultaneous improvement, expanding productivity, and reducing the repetitions of errors. Monitoring investigation data will help in understanding types of incidents such as OOS and OOT and underlying their root causes in your organization. Based on the developed and curated data, we can classify the incidents/events and generate actionable insights.
2. Relying on human error as a root cause
Human error may be a root cause category, but it is neither a specific root cause nor does it lead to reasonable actions. It is to be understood, the main reason is usually in other areas, such as methodology, training, condition, or instruments. It is imperative to find a valid, underlying root cause and to portray it in actionable terms to prevent repetitions and drastically lessen the number of future human-related mistakes.
3. Not getting to the probable root cause
There are numerous reasons where the root cause is not obvious. A few primary reasons could be lack of adequate time and resources, lack of proper training for investigators, and lack of technical and knowledge skill sets.
There are numerous reasons where the root cause is not obvious. A few primary reasons could be lack of adequate time and resources, lack of proper training for investigators, and lack of technical and knowledge skill sets.
4. Generating a cumbersome and unspecific investigation report
The investigation and its report should be clear and simple and can be easily followed up with actions. The report should be supported by supporting facts and scientific rationale so that it can be used as a reference guide in the near future.
5. Overlooking supporting factors for Errors
In the process of finding root cause analysis, supporting factors are often ignored. Supporting factors also require careful investigations and should be included in CAPAs. Utilization of the 5-Whys tool is a valuable tool to recognize supporting factors from root causes.
6. In effective CAPA implementation strategy
Even after implementation of CAPAs, if errors or quality issues reoccur, it shows that root cause analysis is not appropriate and the execution mechanism is cumbersome. Therefore, CAPA’s efficiency should be monitored by the Quality Assurance department, and appropriate actions should be taken.
7. Lack of Human interaction by Investigators
Too many investigations fail to obtain information from the employees with the most relevant insight and information surrounding the event, interviews should be conducted as soon as possible after an event occurs. The essential details of interviews should be summarized in the investigation.
Now that you know this: – hopefully you are in position to answer the Assignment(s)
Do your QA department regularly monitor OOT/OOS/OOC/Incidents? Do they call for insights and discussions with QC/Analytical departments?
What percentages of incidents/OOS are attributed to human errors in CAPA? This percentage is increasing or decreasing year on year basis?
Do your QA/QC department completely use tools such as 5-whys to completely investigate root cause analysis? Do staff has sufficient experience to investigate Root cause analysis?
Do your QA/QC department sufficiently monitor CAPA efficiency? How they are measured?