Any organization that wants to make sure that its processes, procedures, and results are well understood and accurately documented needs to have good documentation practices. This post will list and point out frequent errors/mistakes that should be avoided while writing documents. Additionally, avoiding these errors will guarantee consistency and correctness in the documentation process.
Avoid the following frequent documentation errors:
- Avoid fraudulent data: The most common way that fraudulent data entry happens is when data are “guessed” at or incorrectly entered into a record because they fit within the accepted parameters of the procedure.
- Avoid fraudulent signatures, this happens when a performer/Analyst signs or uses another person’s signature as a verifier.
- Avoid de-linking of documents generated from automated systems to relevant documents. Original data printouts produced by any automated system should be directly linked to the relevant document.
- Avoid back dating of records/documents: Real time documentation should be encouraged to avoid data fabrication. Records that have been backdated shouldn’t be accepted.
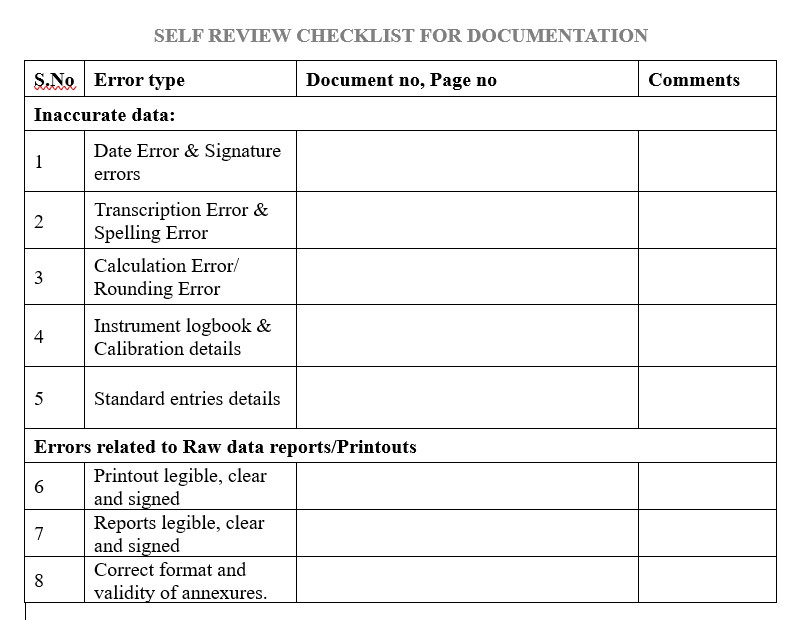
- Avoid inaccurate data entries such as Date Error (DE), Spelling Error (SP), Calculation Error (CE)..etc
- Changes/Errors/Corrections not dated: Any changes to the document must be initiated, dated and explained in legible format according to your organization’s requirements.
- Avoid usage of ditto mark. Original data must always be entered on documents. Substituting ditto marks for original data entries is not allowed.
- Avoid usage of correction fluid, erasers, or tape, pencil: All documentation should be made with an indelible marker according to company standards. Changes to documents should be made according to company standards; changes should be initialed, dated and explained.
- Avoid not more than three corrections on a single page, in case of more than three corrections, whole page shall be strike diagonally; word “Cancelled” shall be written and counter signed by QA personnel.
- Avoid recording information on a separate piece of paper or loose sheet of paper. Always use bound books and ensure the pagination and all pages to be numbered. When portions of a page or a complete page remain unused, a single line must be drawn angularly across the unused portion. Don’t remove any pages or portions from a note book.
- Avoid computer printouts which fade away: Computer print outs taken on thermal paper or easily fading inks should be photocopied and maintained.
- If the original record becomes Illegible and extremely difficult to read or damaged, do not discard. Inform QA/supervisor and transcribing the data to a clean record sheet and attaching the original record sheet. Provide an explanation for the transcription.
What happens if I’m wrong?
Here’s how to handle making a mistake, which everyone will occasionally do:
- Correct the error.
- Draw a single line that crosses the error.
- Enter the accurate data in close proximity to the error.
- Put your initials and the date next to the updated details.
Now that you know this:- hopefully you are in position to answer the Assignment(s)
Does your lab offer appropriate, regular training on best practices for documentation?
Does your lab offer appropriate checks and balances together with solid documentation practices? Name three such procedures.
How does your lab make sure that all documents are properly reviewed to prevent errors such as missing signatures, incomplete entries, and other typographical errors? Do you have sufficient manpower?
What percentage of your documents is Right First Time? (Documents which are approved by QA department without any corrections or rework?)
We hope that after reading this article, you will see the significance of documentation errors and how to prevent them with the right checks and balances.