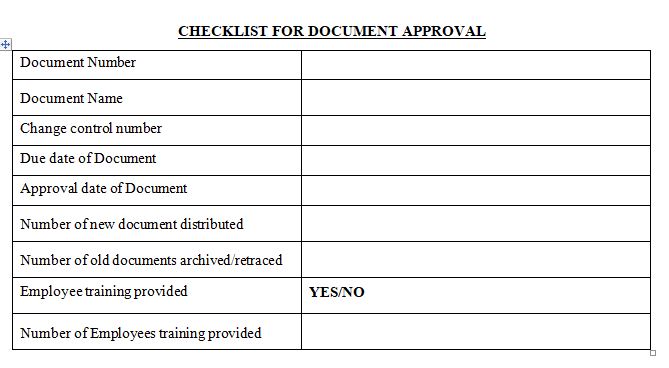
For any organization to guarantee that its activities are carried out correctly and efficiently, good documentation practices are vital. They support the safe, easy retrieval, correct storage, and sharing of information. Good Documentation Practices are founded on five Pillars. This post will examine five essential concepts that can support businesses in keeping correct records and operating at maximum effectiveness.
Truth: This standard maintains that the person who completed the process/task completes the appropriate documentation. All important information that is generated during an operation or process must be recorded by who performed the operation and when it was carried out, such information should be legible, accurate, dated, traceable, and accessible.
Accuracy: Accuracy means that the data is free from Errors/Corrections and Omissions. Any change in entries shall be made so as not to obscure the original entry, shall indicate the reason for such change, and shall be dated and signed or identified at the time of the change.
Completeness: All the data generated from the processes must be included in the document. Data entries must be made or completed at the time the action is performed. Entries in logbooks should be done in chronological order. It is necessary to document anything that directly impacts a product. Record every procedure you write and test performed.
Legibility: The information must be easily recognizable— the data can be read and understood by someone other than the person who wrote it. Original documents should be easily distinguishable from photocopies, and should have clear and concise information. Good documents should have sufficient space for entries, to record variable information and signature and to attach print-outs etc
Timeliness: All entries must be written on the document at the time that the process or activity happened. Further, all entries must be written down in the order that they occurred. All data entries shall be dated on the date of entry and signed or initialed by the person entering the data.
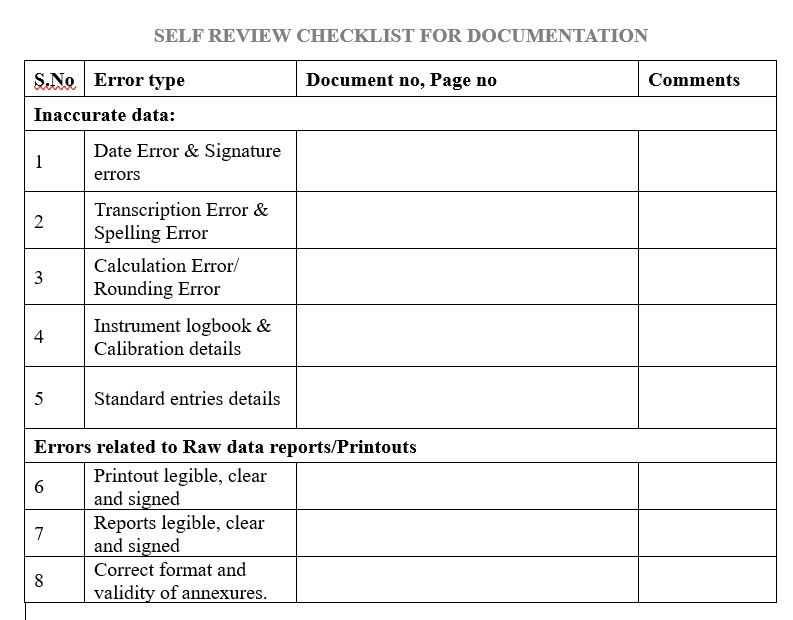
Inaccurate data: These types of errors include but not limited to:
Date Error (DE)
Spelling Error (SP)
Calculation Error (CE)
Entry Error-Including transcription and transposition errors (EE)
Late Entry (LE)
Significant Figure Error (SF)
Rounding Error (RE)
What happens if I’m wrong?
Here’s how to handle making a mistake, which everyone will occasionally do:
- Correct the error.
- Draw a single line that crosses the error.
- Enter the accurate data in close proximity to the error.
- Put your initials and the date next to the updated details.
Now that you know this:- hopefully you are in position to answer the Assignment(s)
Do you have SOP for good documentation practices?
Does your lab provide proper and frequent training for good documentation practices?
Do your lab provide ensure good documentation practices with proper checks and balances? Specify 3 such practices?
How does your lab ensure proper review of documents to avoid incomplete entries, signature missing and other types of errors?
After reading this blog, we hope you can understand the importance documentation errors and to avoid documentation errors with proper checks and balances.