Before implementing an OOT procedure, one must decide the type of OOT of interest. The approach for identifying an OOT depends on this definition. As discussed previously, two main types of OOT definitions exist:
- A result is OOT if it is at odds with previous test results for that batch (comparison within batch) or
- If the result is not similar to the results that past stability studies generated at that same time point (comparison with other batches).
Once a definition is agreed upon, a decision procedure to identify OOT results must be determined.
A Guide for identification of OOT results:
- A Clear Standard operating procedures (SOP) shall be defined, which shall include-selection of batches, samples, test stations, upper and lower limits.
- Although for reliable results greater batches is required, however for practical purposes, most likely around 25 to 30 batches data shall be compiled for fixing the Trend range.
- Results thus obtained from the selected 25 to 30 batches shall be tabulated, which include average mean value; minimum and maximum values are noted.
- Statistics includes: Average values, minimum, maximum, Standard deviation will be calculated for these batches. Excel spread sheet shall be used for Standard deviation calculation.
- A validated Excel sheet, if not calibrated scientific calculator shall be used.
- Maximum and minimum limits shall be taken as the Trend range for upper and lower limits by using 3 sigma method.
- Results that are obtained out of this range (that is upper and lower limits) will be considered as Out of Trend (OOT) value or Outlier value.
- Fixing Specification: However if specification of the product consist of only Not more than, then only Maximum limit for trend can be considered. Minimum limit is not included.
- Fixing Specification: However if specification has defined tolerance limit then both the Maximum and Minimum limits for trend should be considered.
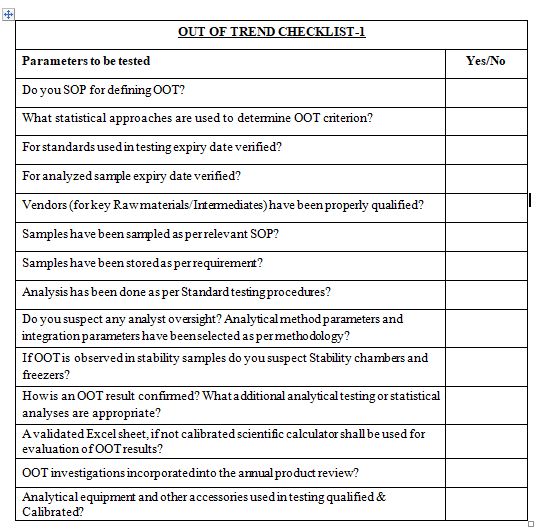
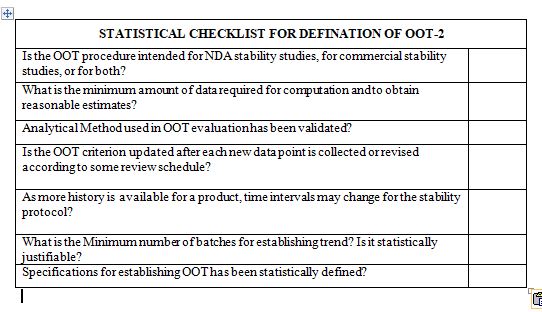
After reading this blog, we hope you can understand the importance of computer system validation and properly use the proposed checklist and improve on it further.
Now that you know this:- hopefully you are in position to answer the Assignment(s)
How does one verify an OOT result? Which extra statistical analysis or analytical testing is necessary?
What role do OOT investigations play in the yearly product review?
What minimal amounts of data required for establishing OOT? If the minimal amount of data is not met, what assessment is carried out?