Out of trend data is a common occurrence in various industries, including pharmaceuticals, food and beverage, and manufacturing. It describes a departure from the standard or accepted practice in a specific procedure or output. Serious repercussions could include product recalls, production delays, and monetary losses. To stop an out-of-trend event from happening again, you need to find its underlying cause. In this article, we will discuss the 12 most likely root causes for out of trend and provide insights on how to address and prevent them. By understanding these root causes, industries can take proactive measures to minimize the occurrence of out of trend events and maintain the quality of their products and processes.
The following are typical possible sources and mechanisms for OOT events that may occur during stability evaluation:
Sample related:
- Completion of expiry date of Intermediate/Key Raw materials.
- Quality attributes change of critical reagents of Vendor and lot variation.
- Selection of sample’s for analysis and sample handling errors by the analysts
- Stability of test samples or critical reagents.
Analytical Method related:
- Analytical errors obtained due to Instrument variation and calibration error.
- Oversight of the Analyst in not following the Standard Testing procedures (STP)
- Changes or variations in standards or reference materials used in the analytical method.
- Analytical method parameters are correct, Integration parameters are correct.
- Oversight of the Analyst in dilution, preparation and execution of the sample.
Environmental and External Factors:
- Temperature, reaction time, and pH effects errors.
- Sample storage conditions like primary and secondary packing materials.
- Working condition of Stability chambers and freezers in which sample are stored.
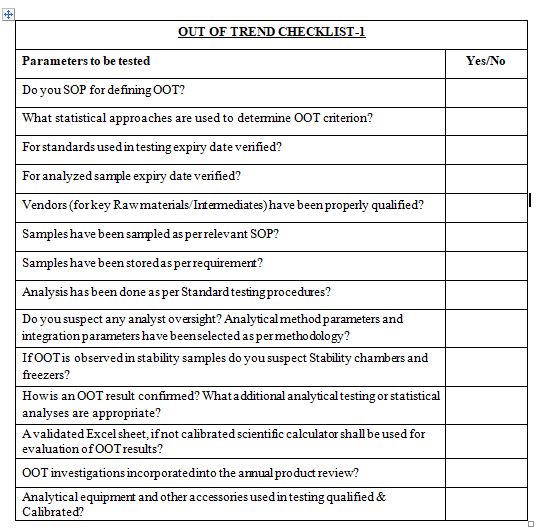
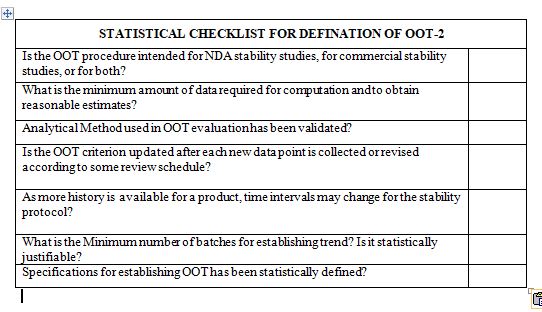
After reading this blog, we hope you can understand the importance of computer system validation and properly use the proposed checklist and improve on it further.
Now that you know this:- hopefully you are in position to answer the Assignment(s)
How does one verify an OOT result? Which extra statistical analysis or analytical testing is necessary?
What role do OOT investigations play in the yearly product review?
What minimal amounts of data required for establishing OOT? If the minimal amount of data is not met, what assessment is carried out?